Introduction:
The bone marrow plasma cell (BMPC) clone burden is typically low in light chain (AL) amyloidosis and some patients do not have a detectable serum monoclonal spike (M-spike). Increased BMPC% and serum free light chain (FLC) are associated with poorer outcomes. However, the outcomes of patients with AL amyloidosis based on the presence or an absence of a measurable serum M-spike before autologous stem cell transplantation (ASCT) has not been explored.
Methods:
This was a retrospective review of patients who had a diagnosis of AL amyloidosis and received ASCT between March 1996 and September 2017. The serum M-spike was recorded before ASCT and patients were divided according to the presence or absence of a measurable serum M-spike. Progression-free survival (PFS) was defined as time from ASCT to disease progression, relapse or death of any cause. Overall survival (OS) was calculated from time of ASCT to death of any cause. Univariate and multivariate analysis for PFS and OS were done using the following variables: age>65 vs. ≤65 years, Mayo 2012 stage 3/4 vs. 1/2, BMPC ≥ 10% vs. <10%, organs involved >2 vs. ≤2, melphalan conditioning 200mg/m2 vs. 140 mg/m2 ,ASCT year >2010 vs. ≤2010, and presence vs. absence of a measurable serum M-spike.
Results:
Seven-hundred and sixteen patients were identified and 521 (73%) had a measurable serum M-spike. Patients who had a measurable serum M-spike were more likely to have BMPC≥ 10% than patients without a measurable serum M-spike (46% vs. 34%, P=0.002) and they were more likely to have a difference in FLC ≥18 mg/dl (47% vs. 29%, P=0.0001).
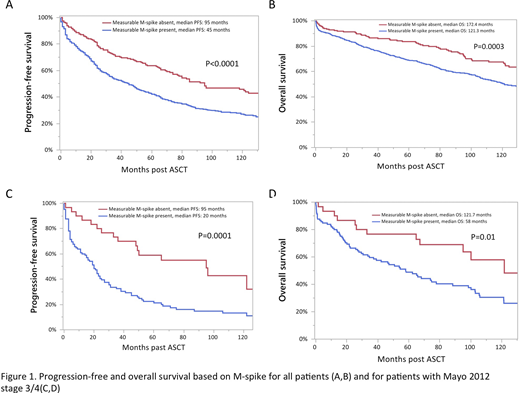
Overall, PFS and OS were significantly shorter in patients who had a measurable serum M-spike compared to patients without a measurable serum M-spike (Figure 1 A,B). The shorter survival was irrespective of the administration of induction therapy before ASCT. We also evaluated the difference in survival in patients with Mayo 2012 stage 3/4 based on the presence or absence of a measurable serum M-spike. The PFS and OS were also significantly different in these patients (Figure 1 C,D).
Predictors for PFS included Mayo 2012 stage 3/4 vs. 1/2 (hazard ratio (HR): 1.5, P=0.003), BMPC ≥ 10% vs. <10% (HR: 1.4,P=0.004), melphalan conditioning 200mg/m2 vs. 140 mg/m2 (HR:0.6, P=0.001), ASCT year >2010 vs. ≤2010 (HR: 0.8, P=0.03), and the presence vs. absence of a measurable serum M-spike (HR: 1.82, P<0.0001). For OS, Mayo 2012 stage 3/4 vs. 1/2 (HR: 2, P<0.0001), melphalan conditioning 200mg/m2 vs. 140 mg/m2 (HR:0.4, P<0.0001), ASCT year >2010 vs. ≤2010 (HR: 0.6, P=0.002), and the presence vs. absence of a measurable serum M-spike (HR:1.9, P=0.003) were predictive.
Conclusion:
The presence of a measurable serum M-spike before ASCT is a negative independent predictor for PFS and OS in AL amyloidosis.
Sidiqi:Amgen: Honoraria; Celgene: Honoraria, Other: Travel grant; Janssen: Honoraria. Dingli:Karyopharm Therapeutics: Research Funding; Apellis: Consultancy; Alexion: Consultancy; Janssen: Consultancy; Bristol Myers Squibb: Research Funding; Rigel: Consultancy; Millenium: Consultancy; Sanofi-Genzyme: Consultancy. Kapoor:Amgen: Research Funding; Celgene: Honoraria; Cellectar: Consultancy; Janssen: Research Funding; Takeda: Honoraria, Research Funding; Sanofi: Consultancy, Research Funding; GlaxoSmithKline: Research Funding. Dispenzieri:Intellia: Research Funding; Alnylam: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; Celgene: Research Funding; Janssen: Research Funding. Kumar:Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Tenebio: Other, Research Funding; Genecentrix: Consultancy; MedImmune: Research Funding; Cellectar: Other; Dr. Reddy's Laboratories: Honoraria; Merck: Consultancy, Research Funding; Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Novartis: Research Funding; Adaptive Biotechnologies: Consultancy; Kite Pharma: Consultancy, Research Funding; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Karyopharm: Consultancy; BMS: Consultancy, Research Funding; Carsgen: Other, Research Funding; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Sanofi: Research Funding. Gertz:Proclara: Other; Abbvie: Other; DAVA oncology: Speakers Bureau; Aurora Bio: Other; Prothena: Other: personal fee; Teva: Speakers Bureau; Physicians Education Resource: Other: personal fee; Annexon: Other: personal fee; Ionis/Akcea: Other: personal fee; Sanofi: Other; Appellis: Other: personal fee; Amgen: Other: personal fee; Janssen: Other: personal fee; Spectrum: Other: personal fee, Research Funding; Medscape: Other: personal fee, Speakers Bureau; Research to Practice: Other; Alnylam: Other: personal fee; Johnson and Johnson: Speakers Bureau; Springer Publishing: Patents & Royalties; Celgene: Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal